Becoming a Non-GMO Project Approved Laboratory

Thank you for your interest in becoming a Non-GMO Project Approved Laboratory.
Non-GMO Project Approved Laboratories are the cornerstone of our Product
Verification Program and are part of a select group of laboratories worldwide permitted to analyze High-Risk Inputs for use in over 57,000 Non-GMO Project Verified Products.
Benefits
All Non-GMO Project Approved Laboratories receive exclusive on-going benefits including:
A dedicated page on the NGP website promoting their expertise and services
Rights to use the Non-GMO Project Approved Laboratory logo
An Approved Laboratory Certificate to confirm their status as a Non-GMO Project Approved Laboratory
When first approved, laboratories also receive these promotional benefits:
A press release announcing their newly approved status published on nongmoproject.org
Notifications sent to all Technical Administrators advising them that a new laboratory has been approved
Requirements
Requirements:
Below is a brief overview of the requirements.
1. ISO Accreditation certificate and scope
2. Details regarding GM Event Tests offered
3. Analytical Reports templates
4. Proficiency Testing participation and results
5. The Non-GMO Project Approved Laboratory Agreement
6. The Non-GMO Project Approved Laboratory Questionnaire
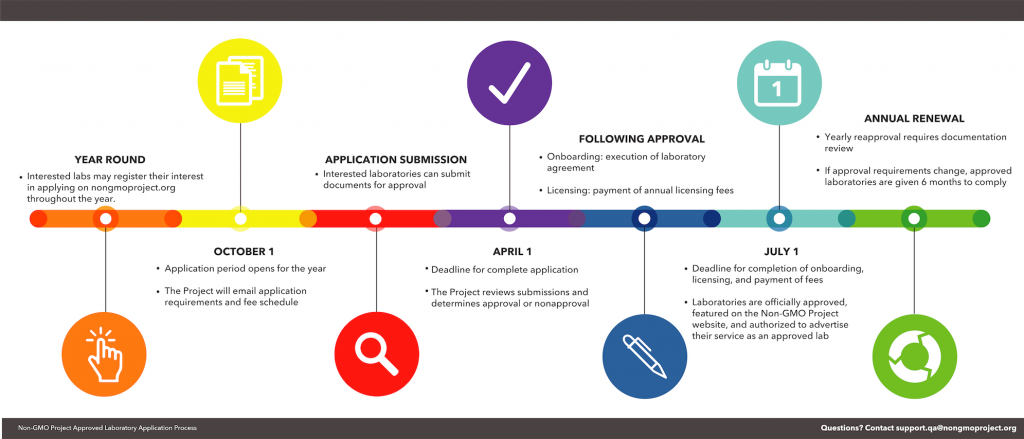
Approval Process
Interested in applying? Review the requirements and register as a lab candidate. Questions about the application? Get in touch with us.
Apply Now
We will help you with any requirement that is unclear after the initial submission of materials has been completed. Please contact us for additional requirements and guidelines.
Learn more about the approval process
Contact the Non-GMO Project
